European Specialty Li
According to the \"European Patent Convention\", all technical inventions can apply for European patents. The European patent can take effect in the contract of the European Patent Convention in accordance with the request of the applicant. In addition, according to the European Patent Agency (EPO) and a separate agreement with a specific country, applicants can also request European patents to take effect in extending countries and effective countries.
In each country that takes effect, the domestic patent authorized by the European patent department has the same effect and is protected according to domestic law.
Website: www.epo.org ■ Tel: +49 (0) 89 2399 4500 (Munich) +49 (0) 30 25901 4500 (Berlin) ■ European patent can take effect The State of the European Patent Convention 2. Extending the country (2 countries) 3. Effective country European patent authorization conditions ● ● is creative, that is, general technical personnel in related technical fields are not obvious; ● Be able to industrialized production or use; ● Fully disclosed so that technical personnel in related technical fields can implement the invention; ● A group of inventions that can only involve one invention or belong to the same invention, but in EPO, you can submit a sub -application application; No It belongs to the object that does not grant patents, such as the treatment or diagnosis of humans or animals, new plants or animal varieties, the substantial biological methods, discovery, mathematics methods, computer software, business methods, etc.; The implementation of the invention shall not violate public order or public ethics, such as clone human body or human embryo for business purposes. 3. Program There are many ways to submit the application directly to the EPO. The online application includes the use of online application software, New online application CMS system ● EPO check the integrity of the application file, and the qualified application will be confirmed by the application date. 3. Forced retrieval EPO will retrieve it according to power, and the retrieval application is novel and creative. After the search is completed, the EPO will produce a search report and provide preliminary review opinions to the applicant. ● 4. Public application The applicant shall determine whether to request a substantial review within 6 months after disclosure. During this period, the applicant should pay the specified fee, extension fee and effective fee for the prescribed. 5. Third -party opinions 6. The substantive review and authorization ● The validity period of the authorization patent is 20 years from the application date, but the annual fee must be paid according to law. \" Five Bureau Patent Examination Highway (IP5 PPH) project \" 7. After the domestic effectiveness After the authorization decision is made public, in general, the authorized European patent must be effective in the designated state, extending the country, and the effectiveness of the country to perform the effective procedures in order to take effect. According to the different provisions of the domestic laws of various countries, applicants may need to pay the necessary fees, submit instructions or translation versions of claims, etc.Effective procedure. However, the United Kingdom, Germany, France, Switzerland, Luxembourg, Monaco, Liechtenstein, Ireland, Albania no longer require domestic procedures and authorize European patents to automatically take effect in these countries. The protection measures for authorized patents are also stipulated by domestic laws. 8. Objury 9. Restricted or revoked 
Europe in Europe The EUROPEAN PATENT Office (EPO) is established in accordance with the European Patent Convention, which is responsible for reviewing the European Patent (EUROPEAN PATENT) that can take effect in 42 countries. The headquarters is located in Munich, Germany. department.
■
+31 (0) 70 340 4500 (Hague) ■
+43 (0) 1 52126 4500 (Vienna)
■ Online consultation
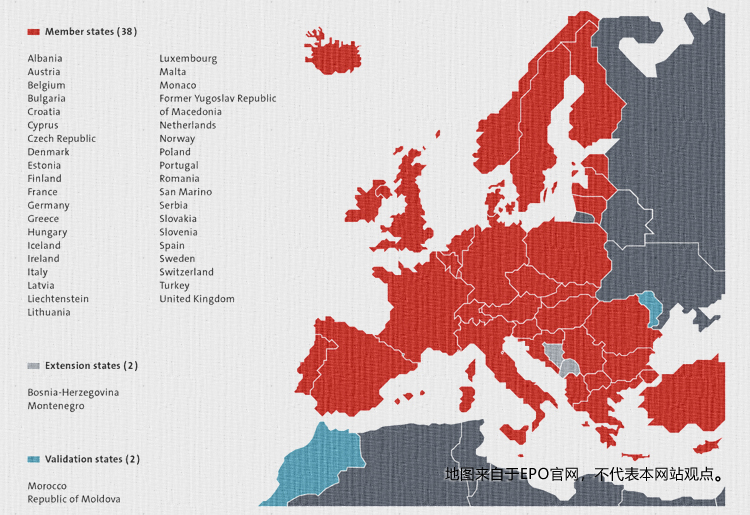 (38 countries)
(38 countries)  In each country that takes effect, the domestic patent authorized by the European patent department has the same effect and is protected according to domestic law.
In each country that takes effect, the domestic patent authorized by the European patent department has the same effect and is protected according to domestic law. ● It has novelty, that is, it does not belong to the existing technology that the public knows in any form before the application date (including the priority date), but within 6 months before the application date (including the priority date) It does not affect novelty or publicity or disclosure of exhibitions at a specific international exhibition;

Applicants who have no residence or business office in Europe need to designate an agent for application procedures.
Application documents include authorized requests, invention instructions, claims, attachment (if any), abstract, priority documents (if any), authorized proxy documents (if.) However, the needs of the EPO official language (English, French, and German) needs to be submitted and translated into one of the above three languages. 
 CHINESE
CHINESE ENGLISH
ENGLISH






